Search results
Search for "NMR titration" in Full Text gives 52 result(s) in Beilstein Journal of Organic Chemistry.
Enhanced host–guest interaction between [10]cycloparaphenylene ([10]CPP) and [5]CPP by cationic charges
Beilstein J. Org. Chem. 2024, 20, 436–444, doi:10.3762/bjoc.20.38
- state of the corresponding host–guest complex. Here, we report on the size complementary formation of the host–guest complex between [10]CPP and the dication of [5]CPP, [10]CPP⊃[5]CPP2+ (Figure 1c). The association constant, Ka, determined by 1H NMR titration, was about 20 times higher than that of the
- 1H NMR titration experiments (see Supporting Information File 1, Figure S1), indicating that the complexation was exergonic with a ΔG = −19 kJ mol−1. This value is about 20 times higher than that of the neutral complex, [10]CPP⊃[5]CPP (Ka = 0.053 × 103 L·mol−1 in TCE-d2 at 50 °C, ΔG = −11 kJ mol−1
Spatial arrangements of cyclodextrin host–guest complexes in solution studied by 13C NMR and molecular modelling
Beilstein J. Org. Chem. 2024, 20, 331–335, doi:10.3762/bjoc.20.33
- delivery [2][3][4], in analytical and preparative chemistry for compound separation [5] and in materials science for small molecule detection [6][7]. Association (binding) constants between the host and guest molecules [8][9][10] are typically measured by 1H NMR titration [11][12] or isothermal titration
A fluorescent probe for detection of Hg2+ ions constructed by tetramethyl cucurbit[6]uril and 1,2-bis(4-pyridyl)ethene
Beilstein J. Org. Chem. 2023, 19, 864–872, doi:10.3762/bjoc.19.63

- analysis of the interaction between the probe and Hg2+ ions To study the solution complexation between G and TMeQ[6], the 1H NMR titration spectrum of TMeQ[6] with different equivalents of guest molecule G was obtained (Figure 8A). When 1.0 equiv G was added, it was found that the proton peak of the G
- molecule partially enters the cavity of TMeQ[6]. The interaction mechanism between the fluorescent probe G@TMeQ[6] and Hg2+ ion was studied using 1H NMR titration experiments (Figure 8B). After the addition of Hg2+ ions to the G@TMeQ[6] system, the proton peaks Ha′, Hb′ and Hc′ on the guest molecules in
- 2.494 × 104 M−1. The interaction ratio between the G@TMeQ[6] probe and Hg2+ was proved to be 1:1 using the molar ratio method and 1H NMR titration spectroscopy. The recognition mechanism may be that there is a competitive effect between the Hg2+ ion and the G molecule, which squeezes out part of G in
Direct C2–H alkylation of indoles driven by the photochemical activity of halogen-bonded complexes
Beilstein J. Org. Chem. 2023, 19, 575–581, doi:10.3762/bjoc.19.42
- absorption spectra recorded in acetonitrile in 1 cm path quartz cuvettes. [DABCO]: 0.5 M; [2a]: 0.5 M. 1H NMR titration of DABCO in a solution of 2a in ACN-d3 to detect their halogen-bonding association through the shift of the signal of Hα. Proposed reaction mechanism for the photochemical alkylation of 1a
Synthesis of a new water-soluble hexacarboxylated tribenzotriquinacene derivative and its competitive host–guest interaction for drug delivery
Beilstein J. Org. Chem. 2022, 18, 539–548, doi:10.3762/bjoc.18.56
- association stoichiometry of the complexes of TBTQ-CB6 with MV, DOX, and SM was found to be 1:1 with association constants of Ka = (7.67 ± 0.34) × 104 M−1, Ka = (6.81 ± 0.33) × 104 M−1, and Ka = (5.09 ± 0.98) × 105 M−1, respectively. The competitive substitution process was visualized by NMR titration. This
- spectroscopy. The competitive binding of TBTQ-CB6 to SM was revealed by NMR titration. In essence, this represents the first application of the convex-concave TBTQ motif for potential use in drug delivery systems. Results and Discussion Synthesis and characterization of host TBTQ-CB6. The host TBTQ-CB6 was
- TBTQ-CB6DOX complex by SM. Similarly, 1H NMR titration experiments were performed to determine the competing effect of SM and the release of DOX from the TBTQ-CB6DOX complex. SM was added to TBTQ-CB6DOX (3.0 mM) in amounts ranging from 0.25 to 2.00 equivalents. As shown in Figure 6, the proton signals
BINOL as a chiral element in mechanically interlocked molecules
Beilstein J. Org. Chem. 2022, 18, 508–523, doi:10.3762/bjoc.18.53

- the thread (see Figure 16). The stereoselective binding of chiral anions by rotaxanes 64a/b was studied by 1H NMR titration experiments, using the dicationic macrocycle (S)-61-Me22+ (obtained by methylation of the triazole units in (S)-61) as a reference system. As guest molecules, the Boc-protected
Tetraphenylethylene-embedded pillar[5]arene-based orthogonal self-assembly for efficient photocatalysis in water
Beilstein J. Org. Chem. 2022, 18, 429–437, doi:10.3762/bjoc.18.45

- of 31% was achieved [30]. This result suggested that m-TPEWP5G-EsY self-assembled nanosystem could be used as a nanoreactor for organic photocatalytic reactions in an aqueous medium. The nature of the interaction between m-TPEWP5G and EsY was evaluated by 1H NMR titration studies in D2O (Figure S5 in
Study on the interactions between melamine-cored Schiff bases with cucurbit[n]urils of different sizes and its application in detecting silver ions
Beilstein J. Org. Chem. 2021, 17, 2950–2958, doi:10.3762/bjoc.17.204

- ]. Results and Discussion The outer-surface interaction of TMeQ[6]-TBT To investigate the outer-surface interaction between TMeQ[6] and TBT, 1H NMR titration is used. As shown in Figure 1, with the addition of TMeQ[6], the proton signals are shifted accordingly. For example, the signals of both Ha, Hb, and
- TBT through outer-surface interaction than the host–guest interaction between the benzene ring or the melamine group of TBT. The host–guest interaction of Q[7]-TBT Using the same method of 1H NMR titration as above. The interaction between Q[7] and TBT is studied and is proved to be different from
- (Supporting Information File 1, Figure S7), and the corresponding binding ability (Ka) is 1.422 × 106 M−1. The host–guest interaction of Q[8]-TBT Since Q[8] has a larger cavity than Q[7], it can bind the entire TBT molecule like Q[7]. However, in the 1H NMR titration experiment (Supporting Information File 1
The fluorescence of a mercury probe based on osthol
Beilstein J. Org. Chem. 2021, 17, 22–27, doi:10.3762/bjoc.17.3
- ), indicating that OST and Hg2+ formed a 2:2 complex. 1H NMR titration analysis of the mode of interaction between the probe and Hg2+ The chemical shift of the resonance peak between the OST probe and Hg2+ in a 1H NMR titration experiment was used to infer the binding mode of the probe and Hg2+. Figure 6 shows
- a 1H NMR titration of OST in the presence of Hg2+ at different concentrations. With the addition of Hg2+, the OST proton resonances shift. At n(Hg2+)/n(OST) = 1, the chemical shift of the proton signals did not change, indicating that the interaction ratio of OST and Hg2+ was 1:1. At this time, the
- of the probe with Hg2+. 1H NMR titration spectra recorded for OST (c = 5 × 10−4 mol∙L−1) during the addition of different molar equivalents of Hg2+ (400 MHz, D2O, n(Hg2+)/n(OST) = 0 (A), 0.2 (B), 0.4 (C), 0.8 (D, E), 1.0 (F), 1.2 (G), pH 7.0, D2O/CH3OH 97:3, v/v). The binding mode of OST and Hg2
Chiral anion recognition using calix[4]arene-based ureido receptors in a 1,3-alternate conformation
Beilstein J. Org. Chem. 2020, 16, 2999–3007, doi:10.3762/bjoc.16.249
- of calix[4]arene immobilised in the 1,3-alternate conformation led to a system possessing a preorganised ureido cavity hemmed with chiral alkyl units in the near proximity. As shown by the 1H NMR titration experiments, these compounds can be used as receptors for chiral anions in DMSO-d6. The chiral
- meta-position of the adjacent aromatic moiety (Figure 4). The complexation ability of the novel receptors towards selected chiral anions was studied using standard 1H NMR titration experiments. There are several reasons why DMSO-d6 was selected as the solvent for the complexation studies: (i) it
- immobilised in the 1,3-alternate conformation resulted in a macrocycle with a preorganised ureido cavity bearing chiral alkyl substituents in the near proximity. As shown by 1H NMR titration experiments, these compounds function as receptors for chiral anions in DMSO-d6. The chiral recognition ability can
Easy access to a carbohydrate-based template for stimuli-responsive surfactants
Beilstein J. Org. Chem. 2020, 16, 2788–2794, doi:10.3762/bjoc.16.229
- the common building block 5 (Scheme 4). Evaluation of amphiphilic properties In order to evaluate the conformational change of compound 19 upon the binding of Zn2+ ions, a 1H NMR titration study was carried out. Thus, a sample of compound 19 was dissolved in acetonitrile-d3 and a spectrum was recorded
- stimuli-responsive surfactants. b) The concept of stimuli-responsive surfactants. 1H NMR titration of compound 19 with Zn2+ ions in acetonitrile-d3. (1) 1:1 Mixture of 1-octanol/H2O, (2) same solvent mixture with compound 19, and (3) same solvent mixture with compound 19 + 1.0 equiv Zn(OTf)2 before
Water-soluble host–guest complexes between fullerenes and a sugar-functionalized tribenzotriquinacene assembling to microspheres
Beilstein J. Org. Chem. 2020, 16, 2551–2561, doi:10.3762/bjoc.16.207

- with fullerenes (Figure 1). All these TBTQ-based hosts were found to bind fullerenes in organic solvents with different strengths, as indicated by UV–vis or 1H NMR titration experiments. Moreover, easily accessible C3v-symmetrical sixfold hydroxy-functionalized TBTQ derivatives gain increasing
NMR Spectroscopy of supramolecular chemistry on protein surfaces
Beilstein J. Org. Chem. 2020, 16, 2505–2522, doi:10.3762/bjoc.16.203

Host–guest interaction of cucurbit[8]uril with oroxin A and its effect on the properties of oroxin A
Beilstein J. Org. Chem. 2020, 16, 2332–2337, doi:10.3762/bjoc.16.194

- the cucurbit[n]uril-guest can be inferred from the chemical shift changes of the guest proton resonance peaks. 1H NMR titration experiments were performed in D2O containing 10% DMSO by volume at room temperature. As shown in Figure 2 and Table 1, upon the addition of Q[8], some of the peaks of the
- −1. The results of the UV absorption spectrum analysis showed that Q[8] enhanced the cumulative release of OA in artificial gastric juice by 2.3-fold, but had no effect on its antioxidant activity. The molecular structure of OA (A) and Q[8] (B). 1H NMR titration of OA with Q[8] were performed in D2O
Design, synthesis and application of carbazole macrocycles in anion sensors
Beilstein J. Org. Chem. 2020, 16, 1901–1914, doi:10.3762/bjoc.16.157

- electrodes showed higher selectivity towards benzoate than acetate, i.e., the selectivity patterns of the final sensors deviated from that predicted by the classic titration experiments. While the binding constants obtained by NMR titration in DMSO-d6/H2O solvent systems provided important guidance for
Disposable cartridge concept for the on-demand synthesis of turbo Grignards, Knochel–Hauser amides, and magnesium alkoxides
Beilstein J. Org. Chem. 2020, 16, 1343–1356, doi:10.3762/bjoc.16.115

- -hydroxybenzaldehyde phenylhydrazone [52] or a mixture of benzoic acid and 4-(phenylazo)diphenylamine [53]. Iodine can also be used [54]. Similar concentrations were obtained by NMR titration with 1,5-cyclooctadiene as a standard (Section 1.2.10 in Supporting Information File 1) [55]. The heat released from the
[3 + 2] Cycloaddition with photogenerated azomethine ylides in β-cyclodextrin
Beilstein J. Org. Chem. 2020, 16, 1296–1304, doi:10.3762/bjoc.16.110
- Zagreb, Croatia 10.3762/bjoc.16.110 Abstract Stability constants for the inclusion complexes of cyclohexylphthalimide 2 and adamantylphthalimide 3 with β-cyclodextrin (β-CD) were determined by 1H NMR titration, K = 190 ± 50 M−1, and K = 2600 ± 600 M−1, respectively. Photochemical reactivity of the
- determine stability constants for the inclusion complexes 2@β-CD and 3@β-CD. The NMR titration for 2 with β-CD was carried out in CD3CN/D2O (3:7 v/v). The addition of β-CD to the solution of 2 induced downfield shifts of the H-signals corresponding to the cyclohexane 2 and 6 positions, as well as H-signals
Preparation of anthracene-based tetraperimidine hexafluorophosphate and selective recognition of chromium(III) ions
Beilstein J. Org. Chem. 2019, 15, 2847–2855, doi:10.3762/bjoc.15.278
- the tertiary amines, and the π systems, were most likely the binding sites for Cr3+ through Cr3+···N and Cr3+···π interactions (Scheme 2). In order to obtain further information on the binding pattern between 3 and Cr3+, 1H NMR titration studies were done in DMSO-d6. The spectra are depicted in Figure
- tertiary amine, did not shift discernibly during 1H NMR titration; (2) if the tertiary amine groups coordinated to one Cr3+ ion each, a 1:2 binding mode would have been determined for 3⋅Cr3+; and (3) from the molecular structure of 3, due to the distance, it is spatially impossible that one Cr3+ ion is
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- 1H NMR titration experiments in CD3CN solution and cyclic voltammetry in 0.1 M TBA·PF6 (TBA = tetrabutylammonium) in CH3CN (Figure 3). WinEQNMR2 analysis of the titration data has revealed that among the tested anions, the receptor 4 binds strongly with chloride and benzoate ions through favorable
- to be very difficult to further proceed. However, the anion binding property of the corresponding bistriazolium macrocyclic part 5·(BF4)2 (Figure 5) has successfully been investigated using 1H NMR titration experiments in CD3CN. The highest binding affinity was found to be almost similar for both BzO
- -derived tris(crown ether) host (eTC) via a dynamic covalent chemistry approach involving triple threading and a Grubbs-catalyzed ring-closing metathesis [68]. 1H NMR titration experiments have been used for the identification of co-conformations of the four related stable states of 19. The authors have
Multiple threading of a triple-calix[6]arene host
Beilstein J. Org. Chem. 2019, 15, 2092–2104, doi:10.3762/bjoc.15.207

- ); (c) DOSY spectra of 6 (black line) and a 1:1 mixture of 6 and 7+·TFPB− (red line). Inset: structures of the triple-calix[6]arene 6 and 7+6 pseudo[2]rotaxane obtained by molecular mechanics calculations. 1H NMR titration of 6 with 7+·TFPB– (CDCl3 , 298 K, 600 MHz). Significant portions of the 1H NMR
- molecular mechanics calculations. (a–d) 1H NMR titration of 6 with 4+·TFPB− (CDCl3, 298 K, 600 MHz). Significant portions of the 1H NMR spectra of: (a) 6; (b) 1:1, (c) 1:2, (d) 1:3, mixture of 6 and 4+·TFPB–. (e) HR-ESI-FT-ICR mass spectrum of 4+6. (f) HR-ESI-FT-ICR-CID mass spectrum of (4+)26. Different
- obtainable by triple-threading of 6 with 8+. (Left) HR-ESI-FT-ICR mass spectrum of 8+6. (Right) HR-ESI-FT-ICR-CID mass spectrum of (8+)26. (a–d) 1H NMR titration of 6 with 8+·TFPB− (CDCl3 , 298 K, 600 MHz). Significant portions of the 1H NMR spectra of: (a) 6; (b) 1:1, (c) 1:2, (d) 1:3, mixture of 6 and 8
Complexation of chiral amines by resorcin[4]arene sulfonic acids in polar media – circular dichroism and diffusion studies of chirality transfer and solvent dependence
Beilstein J. Org. Chem. 2019, 15, 1913–1924, doi:10.3762/bjoc.15.187
- fact, they reveal important information about the thermodynamic and kinetics of the complexation, that cannot be obtained based on standard 1H NMR titration (because there are no complexation-induced shifts). We rationalize these results assuming that in [1(R)-2]DMSO solution the complex partially
Complexation of 2,6-helic[6]arene and its derivatives with 1,1′-dimethyl-4,4′-bipyridinium salts and protonated 4,4'-bipyridinium salts: an acid–base controllable complexation
Beilstein J. Org. Chem. 2019, 15, 1795–1804, doi:10.3762/bjoc.15.173

- by the additional multiple hydrogen-bonding interactions between the hosts and the guests, which were evidenced by 1H NMR titration, X-ray crystal structures and DFT calculations. Moreover, we also found that the Ka values of the complexes could be significantly enhanced with larger counteranions of
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
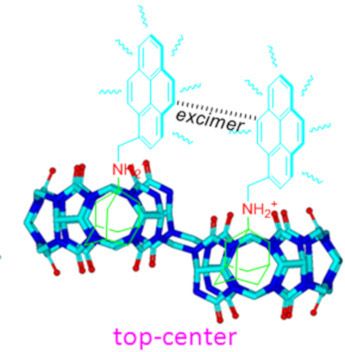
- cavities with homotropic allostery effect. In the case of the 1H NMR titration experiments for host–guest interactions of G1·host-2, the chemical shift changes in G1 with increasing host-2 concentration are similar to those observed in the host-1 systems. However, the largest difference in the host–guest
- ad host-2 were further studied by UV–vis spectra (Supporting Information File 1, Figure S3) and Job plot (Supporting Information File 1, Figure S4). In order to obtain detailed information on the mechanism of the complexation of G2 with host-1 and -2, 1H NMR titration experiments in acidic aqueous
Protein–protein interactions in bacteria: a promising and challenging avenue towards the discovery of new antibiotics
Beilstein J. Org. Chem. 2018, 14, 2881–2896, doi:10.3762/bjoc.14.267

- -transfer difference NMR (STD-NMR) led to the identification of thirty fragments. Subsequent 2D-15N–1H HSQC NMR returned four fragment hits 28–31 (Figure 7), with binding affinities, determined by NMR titration, in the low millimolar range. Of all of the fragments, tetrazole 31 was chosen for further
Calixazulenes: azulene-based calixarene analogues – an overview and recent supramolecular complexation studies
Beilstein J. Org. Chem. 2018, 14, 2488–2494, doi:10.3762/bjoc.14.225

- characterization. This finding suggested to us that solution complexation studies with other electron-deficient suitable guests could be conducted in dichloromethane (DCM). The concentrations that could be obtained with DCM were too dilute for typical NMR titration studies, but we judged that they could instead be













































































































































































































































